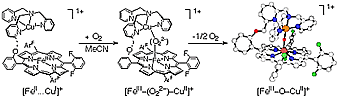Dr. Karlin’s bioinorganic research focuses on coordination chemistry relevant to biological and environmental processes, involving copper or heme (porphyrin-iron) complexes. Of interest are the reactivities with dioxygen (O2), nitrogen oxides (NOx) and organohalide (R-X) pollutants, and metal/O2 chemistries with organic substrates, DNA and proteins. Essential copper or heme-containing enzymes function in O2-transport, electron transfer, O2-reduction (oxidase activity), biological substrate oxygenation (i.e., oxygenase activity), and reduction of NO2-, NO or N2O A variety of novel metal sites are observed in proteins, including those with 1 Cu ion plus cofactor, 2 Cu’s, a cluster of 3 or 4 Cu’s, or porphyrinate-iron/Cu(Fe) centres.
Dr. Karlin’s main approach involves synthetic modeling, i.e. biomimetic chemistry. Research approaches utilized include (a) the rational design and syntheses of ligands allowing formation of appropriate Cu or Fe complexes, (b) elucidation of structure and physical properties using IR, UV-Vis, resonance Raman and EPR spectroscopies, X-ray crystallography, stopped-flow kinetics, magnetic susceptibility and cyclic voltammetry, and (c) reactivity and mechanistic investigations such as in reversible O2-binding by copper complexes, and the oxidation of substrates. The main objective is to establish relevant coordination chemistry of copper or heme-Cu(Fe) systems, to provide a sound basis for deducing biological active site structures, the nature of reactive intermediates, and mechanism. These studies may also provide a rationale for the design of practical O2-binders, organic oxidation and NOx reduction catalysts, or agents which can dehalogenate R-X pollutants.
Recent notable achievements in our labs include:
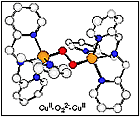
(i) the detailed chemical (e.g., spectroscopy, kinetics of formation) and structural determination of a several types of copper-dioxygen adducts,
(ii) the generation of model systems for cytochrome c oxidase (heme-copper) O2-reactivity, with well characterized m-peroxo FeIII-(O22-)-CuII and m-oxo FeIII-(O2-)-CuII species, and
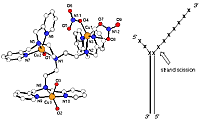
(iii) the demonstration of trinuclear copper complex site-specific oxidative cleavage near the junction of double and single-stranded DNA.
Professor Ken Karlin is also part of the recently awarded Collaborative Research in Environmental Molecular Science (CRAEMS) at Johns Hopkins University.
Research Details:
Biomimetic Studies of Copper Dioxygen Adducts and Chemistry