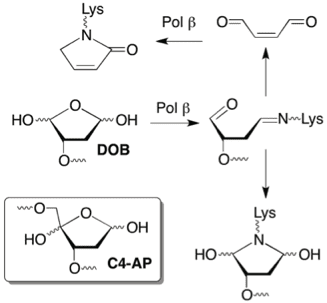
Many damaged nucleotides are repaired by base excision repair (BER). DNA polymerase beta (Pol beta), which is over expressed in many tumor cells, plays a vital role in BER. Pol beta is one of a small number of bifunctional polymerases that possess lyase activity as well as polymerase activity. We determined that two lesions (DOB, C4-AP) that are produced in DNA by potent antitumor agents, irreversibly inactivate Pol beta. Our ability to chemically synthesize oligonucleotides containing DOB or C4-AP greatly facilitated these investigations. Determination that C4-AP and DOB inactivate a repair enzyme that is over expressed in tumors provides chemical insight into why drugs that produce such DNA damage are so cytotoxic and has provided the inspiration for the design of inhibitors of DNA repair enzymes.
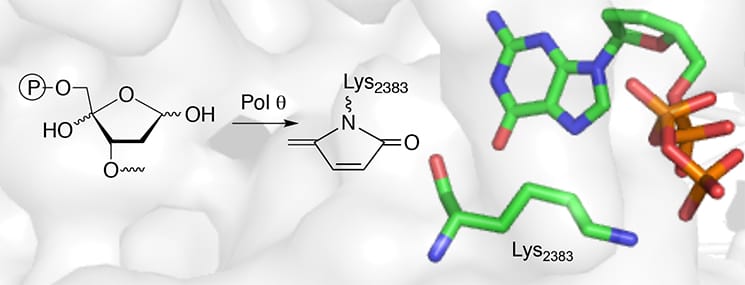
Recently, we demonstrated that DNA polymerase theta (Pol theta), another “bypass polymerase” (also known as a “translesion synthesis” polymerase) is inactivated by C4-AP. Pol theta is of great interest as an anticancer target because it is nonessential in healthy cells but crucial in cancer cells, particularly those that are deficient in double strand break repair. We used this inactivation to identify the nucleophile (Lysine 2383) involved in the lyase activity of Pol theta using mass spectrometry, and demonstrated that Pol theta is unique in that the polymerase and lyase activities are present in the same active site. Furthermore, we were able to demonstrate that lysine 2383 is required for both enzyme functions. These observations could be used to discover inhibitors to this important enzyme.
For relevant publications see:
• Irreversible Inhibition of DNA Polymerase by an Oxidized Abasic Lesion. Guan, L.; Greenberg, M. M. J. Am. Chem. Soc. 2010, 132, 5004-5005.
• Inhibition of Short Patch and Long Patch Base Excision Repair by an Oxidized Abasic Site. Guan, L.; Greenberg, M. M. Biochemistry 2010, 49, 9904-9910.
• Long Patch Base Excision Repair Compensates for DNA Polymerase Inactivation by the C4′-Oxidized Abasic Site. Jacobs, A. C.; Kreller, C. R.; Greenberg, M. M. Biochemistry 2011, 50, 136-143.
•The A-Rule and Deletion Formation During Abasic and Oxidized Abasic Site Bypass by DNA Polymerase Theta. Laverty, D. J.; Averill, A. M.; Doublié, S.; Greenberg, M. M. ACS Chem. Biol. 2017, 12, 1584-1592. (PMC: 5499511)
• DNA Polymerase Inactivation by Oxidized Abasic Sites. Stevens, A. J.; Guan, L.; Bebenek, K.; Kunkel, T. A.; Greenberg, M. M. Biochemistry 2013, 52, 975-983.
•Mechanistic Insight Through Irreversible Inhibition: DNA Polymerase Theta Uses a Common Active Site for Polymerase and Lyase Activities. Laverty, D. J.; Mortimer, I. P.; Greenberg, M. M. J. Am. Chem. Soc. 2018, 140, 9034-9037. (PMC: 6085753)
