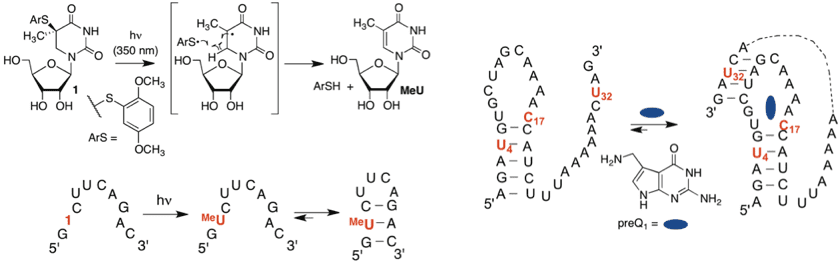
Photoconvertible nucleosides are useful for providing spatiotemporal control over nucleic acid structure and function. We recently reported a novel nucleotide that provides photochemical control over RNA folding. The precursor to 5-methyluridine (1) is novel in that it perturbs duplex formation by disrupting -stacking. Furthermore, 1 yields the native nucleotide within a microsecond following photolysis at 350 nm, which makes it useful for studying folding processes that occur on the sub-millisecond timescale in a time resolved manner. We demonstrated the utility of 1 by photochemically controlling the folding of the preQ1 aptamer.
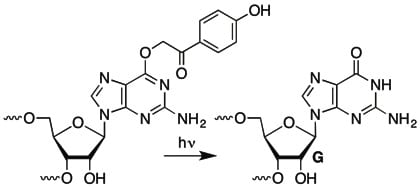
In another example we utilized photochemical release of guanosine to study the rapid kinetics of RNA annealing of a chaperone protein.
For relevant publications see:
•Photochemical Control of RNA Structure by Disrupting -Stacking. Resendiz, M. J. E.; Schön, A; Freire, E.; Greenberg, M. M. J. Am. Chem. Soc. 2012, 134, 12478-12481.
• Light-Triggered RNA Annealing by an RNA Chaperone. Panja, S.; Paul, R.; Greenberg, M. M.; Woodson, S. A. Angew. Chem. Int. Ed. 2015, 54, 7281-7284.