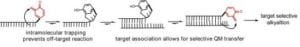
Quinone methides (QM) are highly electrophilic and transient intermediates that can form in vivo as byproducts of metabolism. These intermediates are responsible for DNA alkylation observed after exposure to drugs such as tamoxifen, food additives such as butylated hydroxytoluene (BHT), and certain natural products such as mitomycin C. Our laboratory has capitalized on the reversible nature of ortho-QM intermediates in a design for sequence specific alkylation that is compatible with a cellular environment. Internal trapping of the QM is highly effective at suppressing off-target reaction and allows for its transfer only after association with a chosen target. Applicability of this strategy is boosted by the remarkable resistance of QM transfer to high concentrations of thiols like those present in our cells to protect against damage by electrophiles.
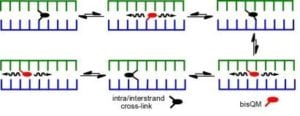
Tandem formation of QM intermediates (bisQMs) provides a basis for highly efficient cross-linking of DNA since their reversible reaction offers an escape from kinetic traps that would otherwise suppress cross-linking. Even after cross-links are established, they still remain dynamic and allow for strand exchange and cross-link migration. The parameters facilitating this migration and their implications for DNA damage and repair are now under investigation.