Development of Novel Hydropersulfide Precursors
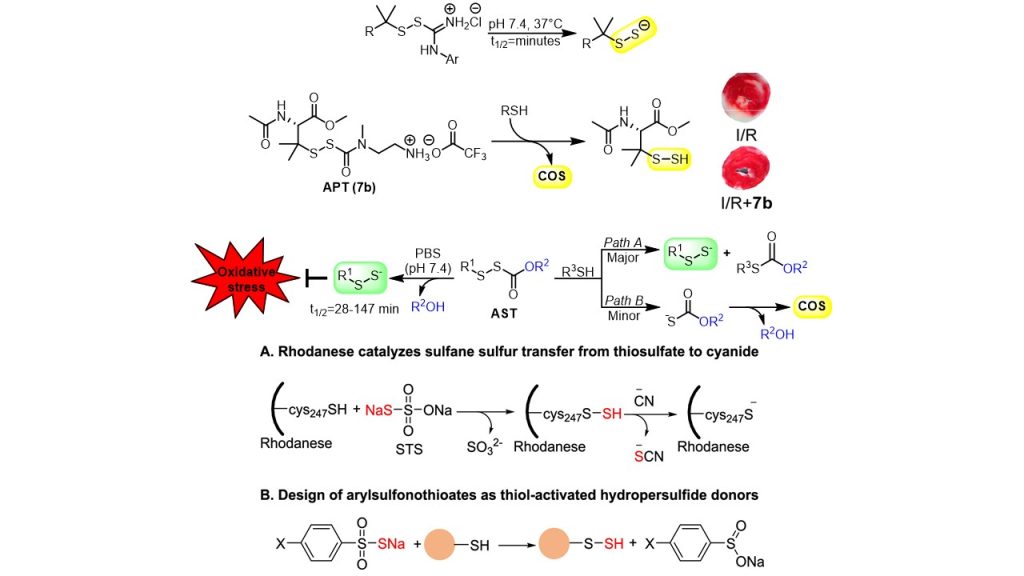
Because of their inherent instability, hydropersulfides (RSSH) must be generated in situ using precursors. We have developed four classes of hydropersulfide prodrugs: S-substituted thioisothioureas (TIU), N-alkylamine perthiocarbamates (APT), alkylsulfenyl thiocarbonates (AST), and arylsulfonothioates (AT). All four classes demonstrate efficient and controllable release of RSSH under physiological conditions and in cardiomyocyte cell models.
In the presence of thiol, alkylamine-substituted perthiocarbamates can also serve as donors of carbonyl sulfide (COS), a precursor to H2S, while the alkylsulfenyl thiocarbonates release COS only as a minor product. In the presence of amines, hydrolysis of the AST precursors to release persulfide dominates over nucleophilic attack at pH 7.4. Arylsulfonothioates are thiol-activated slow-release sulfane sulfur donors superior to sodium thiosulfate, with RSSH release influenced by substituents on the aryl ring.
The self-immolative linker of N-alkylamine perthiocarbamates also improves photorelease of hydropersulfides caged with p-hydroxyphenacyl and 7-diethylaminocoumarin (DEACM) photocleavable groups in vitro and in MCF-7 cancer cells.
Chemical Biology and Therapeutic Applications of Hydropersulfide Precursors
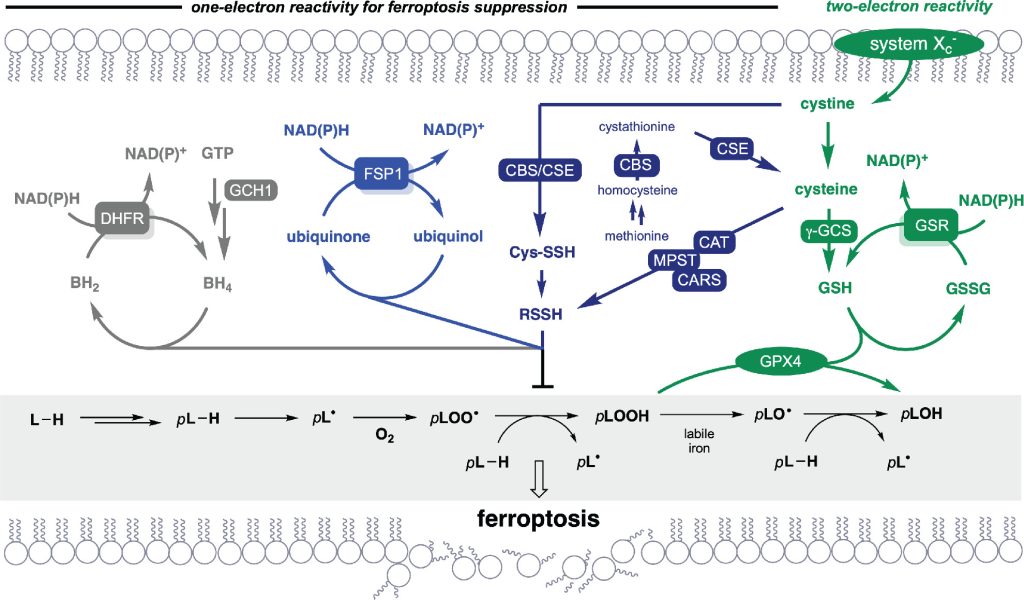
Hydropersulfides are excellent single-electron reductants and hydrogen-atom donors compared to the corresponding thiols, and they are naturally produced intracellularly under oxidative stress conditions. They are thought to induce antioxidant response signaling and protect protein Cys residues from overoxidation.
In collaboration with the Pratt group at the University of Ottawa, we have applied our persulfide donors to the inhibition of ferroptosis, a cell death pathway characterized by excessive lipid peroxidation driven in part by iron-mediated Fenton chemistry. Using the fluorescence-enabled inhibited autoxidation (FENIX) assay, alkyl hydropersulfides were found to have outstanding dose-dependent lipid peroxyl trapping kinetics in liposomes. The faster-releasing APT derivatives proved to be the most potent among the persulfide donors tested. These results were validated in cells through rescue experiments under GPX4-knockout-induced ferroptosis.
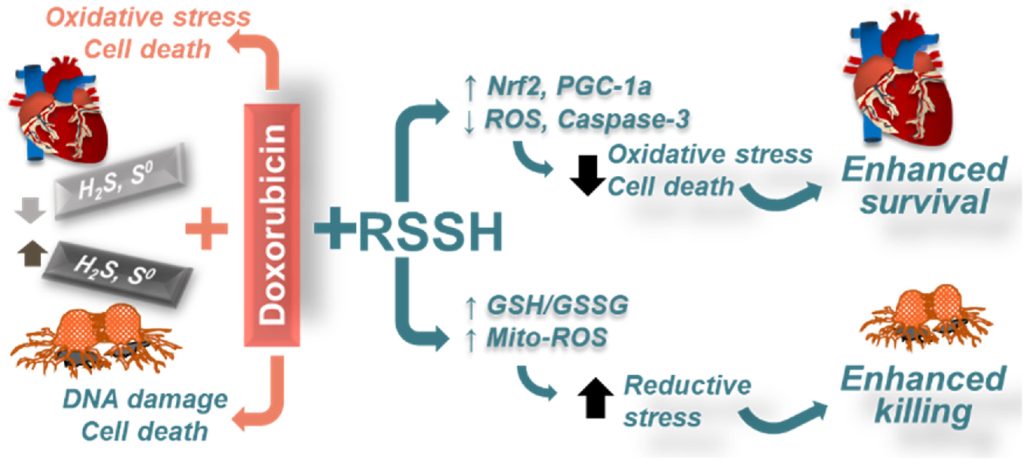
Following Langendorff studies conducted with APT 7b (see above section) showing that treatment is cardioprotective against myocardial ischemic/reperfusion injury, we examined the efficacy of persulfide precursors against doxorubicin-induced cardiotoxicity (DIC). This is an adverse effect thought to occur via direct ROS generation from single-electron chemistry, aberrant iron accumulation and other mechanisms. The slow-releasing AST persulfide precursor was found to provide superior dose-dependent protection against DIC-associated ROS in cardiomyocytes via Nrf2 and PGC-1α related signaling. Our persulfide precursors increased the sensitivity of cancer cells to doxorubicin by depletion of oxidized glutathione in mitochondria, resulting in reductive stress due to elevated basal levels of reducing equivalents in cancer cells.
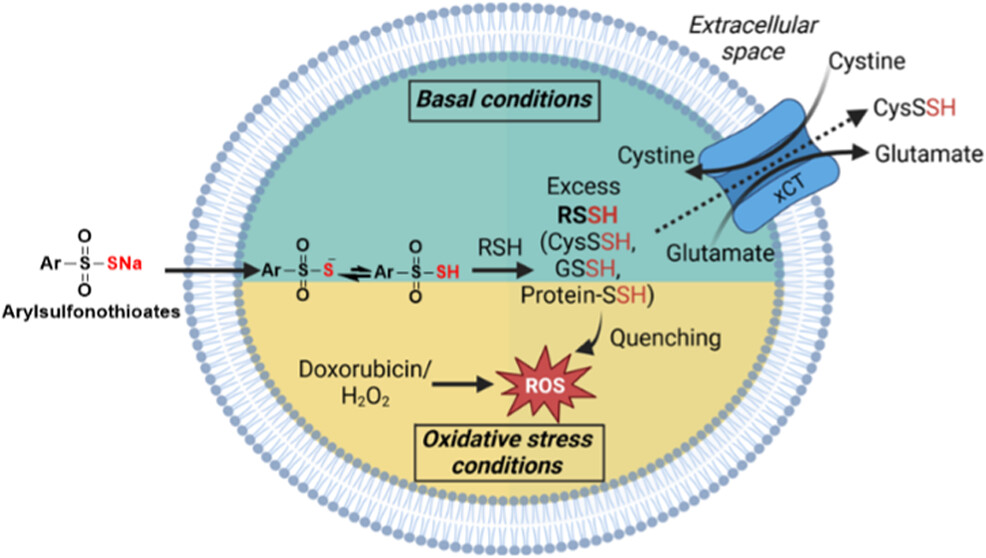
We are interested in mechanisms of reactive sulfur species homeostasis and cellular responses to RSSH elevation. Using arylsulfonothioates as mimics of gradual intracellular RSSH biogenesis, we have demonstrated that cysteine persulfide (CysSSH) export through xCT, the cystine-glutamate antiporter, responds dynamically to the redox state of H9c2 cardiomyocytes. Treatment with AT precursor 1g resulted in xCT-dependent elevation of extracellular CysSSH, while challenge with oxidative stress significantly decreased CysSSH export. We believe that modulation of RSSH export buffers cells against reductive stress under basal conditions while retaining RSSH when needed for antioxidant response.
Nitroxyl (HNO) Reactivity with Reactive Sulfur Species
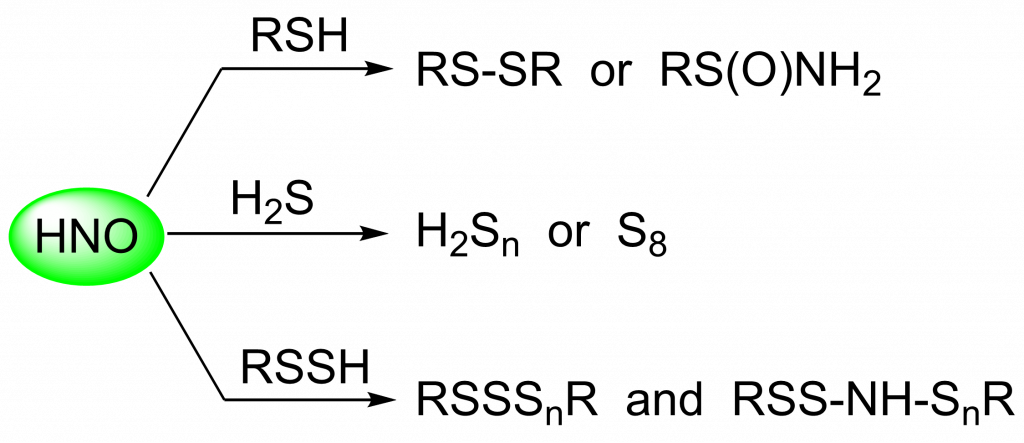
Previous work in our lab focused on the reaction of HNO with thiols. We published an expansion of these studies with other reactive sulfur species: H2S and RSSH. The reaction of HNO with H2S produces either hydrogen polysulfides (H2Sn) or S8 depending on their relative concentrations. Both S8 and H2Sn are believed to be involved in cellular sulfane sulfur homeostasis. In this study, we also confirmed that persulfides exhibit enhanced nucleophilicity compared to thiols, which is the result of more than just differences in pKa. This difference in reactivity provides a possible explanation for the specificity of HNO signaling.
New physiologically useful HNO donors
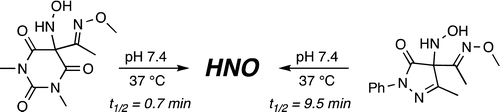
Due to its inherent reactivity, nitroxyl (HNO), must be generated in situ through the use of donor compounds, but very few physiologically useful HNO donors exist. Novel N-substituted hydroxylamines with carbon-based leaving groups have been synthesized, and their structures confirmed by X-ray crystallography. These compounds generate HNO under nonenzymatic, physiological conditions, with the rate and amount of HNO released being dependent mainly on the nature of the leaving group. A barbituric acid and a pyrazolone derivative have been developed as efficient HNO donors with half-lives at pH 7.4, 37 °C of 0.7 and 9.5 min, respectively. PDF
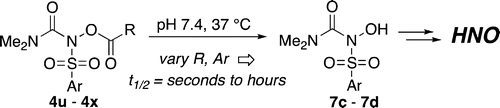
A wide range of N,O–bis-acylated hydroxylamine derivatives with chloro or arenesulfonyl leaving groups, and a related set of N-hydroxy-N-acylsulfonamides, have been synthesized and evaluated for nitroxyl (HNO) production. Mechanistic studies have revealed that the observed aqueous chemistry is more complicated than originally anticipated, and have been used to develop a new series of efficient HNO precursors (4u–4x, 7c–7d) with tunable half-lives. PDF
New HNO Detection Strategies
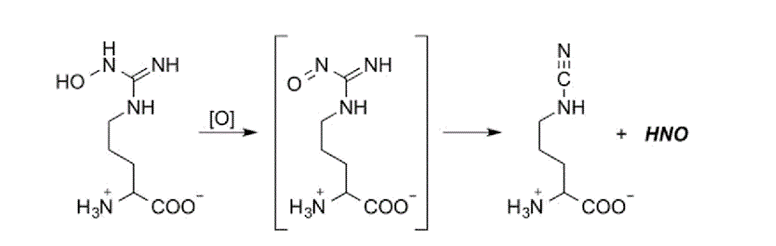
Oxidation of N-hydroxy-L-arginine by excess hypochlorous acid (HOCl) is examined by membrane inlet mass spectrometry and gas chromatography. The major products are determined to be HNO and HNO-derived N2O. The analogous production of HNO from HOCl oxidation of hydroxylamine, hydroxyurea, and (to a lesser extent) acetohydroxamic acid is also observed. PDF
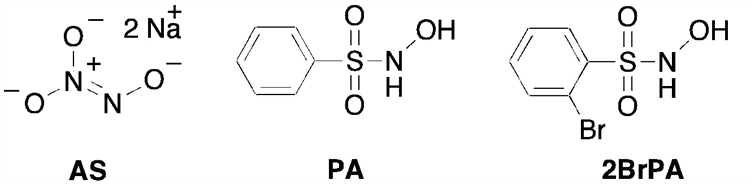
Membrane inlet (or introduction) mass spectrometry (MIMS) was used to detect nitroxyl (HNO) in aqueous solution for the first time. The common HNO donors Angeli’s salt (AS) and Piloty’s acid (PA), along with a newly developed donor, 2-bromo-N-hydroxybenzenesulfonamide (2-bromo-Piloty’s acid, 2BrPA), were examined by this technique. MIMS experiments revealed that under physiological conditions 2BrPA is an essentially pure HNO donor, but AS produces a small amount of nitric oxide (NO). In addition, MIMS experiments also confirmed that PA is susceptible to oxidation and NO production, but that 2BrPA is not as prone to oxidation. PDF
Characterization and reactivity of HNO-derived modifications in peptides and proteins
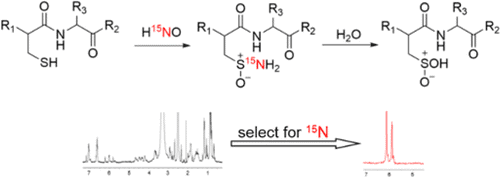
HNO, a potential heart failure therapeutic, is known to post-translationally modify cysteine residues. Among reactive nitrogen oxide species, the modification of cysteine residues to sulfinamides [RS(O)NH2] is unique to HNO. Because this modification can alter protein structure and function, we have examined the reactivity of sulfinamides in several systems, including small organic molecules, peptides, and a protein. At physiological pH and temperature, relevant reactions of sulfinamides involve reduction to free thiols in the presence of excess thiol and hydrolysis to form sulfinic acids [RS(O)OH]. In addition to utilizing ESI-MS and other spectroscopic methods to study sulfinamide reduction, we have applied 15N-edited 1H-NMR techniques to sulfinamide detection and used this method to explore sulfinamide hydrolysis. We have also investigated the effect of local environment on the reactivity of HNO with C-terminal cysteines. HNO has been shown to enhance cardiac sarcoplasmic reticulum Ca2+ cycling independent of the β-adrenergic pathway. In a collaborative project, the effects of HNO on the cardiac protein, phospholamban (PLN) was investigated. PDF PDF
Past and present funding sources:


