Many chemical transformations of energetically excited molecules involve time-dependent crossings between molecular electronic states – or nonadiabatic transitions; these are the result of inextricable coupling between nuclear and electronic motions that prompt changes in molecular structure in concert with relaxation of excited molecular electronic states. In our group we are using a combination of time-resolved spectroscopic methods and supporting computations to elucidate how reactant structure impacts the possibilities, efficiencies, and rates for nonadiabatic transitions that underlie photochemical bond formation and isomerization. The motivation for this work derives from fundamental scientific significance of these couplings in molecular quantum mechanics and the exciting possibility of harnessing nonadiabaticity and couplings between excited states for light-driven applications, such as photoactivated molecular switches/motors and photochemical synthesis.
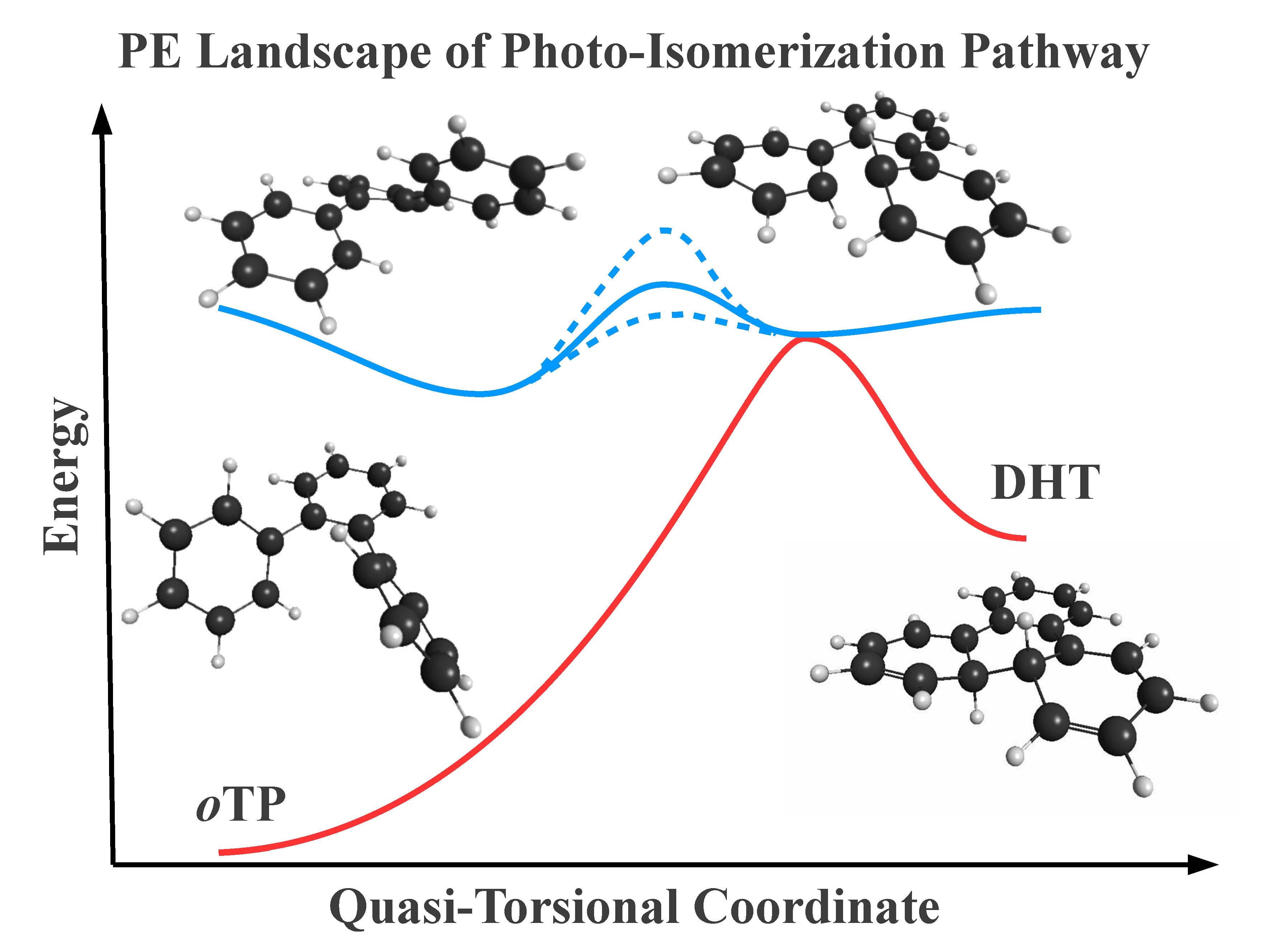
We have been examining structure-dynamics relationships underlying photoinduced 6π electrocyclization in a collection of ortho-arenes, specifically. This is a fundamental process used in photochemical synthesis of polyaromatic hydrocarbons and also underlies the photoresponses of a popular class of molecular photoswitch. Nonadiabatic C-C bond formation is predicted from orbital symmetry correlations between isomers, yet these (Woodward-Hoffman) rules cannot predict structure-dependent topography of the excited- and ground-state energy surfaces that ultimately determine the photochemical reactivity of their excited states or photoproduct stabilities. Simple model systems, like ortho-terphenyl, are ideal for comprehensive evaluation of the impact of reactant structure and chemical environment on photochemical dynamics, as well as for the development of new spectroscopic probes of branching between excited-state deactivation pathways. We have examined how chemical modifications, including intramolecular steric interactions, extension to larger ortho-arenes (4+ rings), and BN doping (in collaboration with Holger Bettinger, U. Tubingen, Germany) impacts photochemical dynamics and reactivity. We seek to understand how these interactions tune the shapes of potential-energy landscapes and therefore identify or design structural motifs controlled for applications in photochemical synthesis or switching.