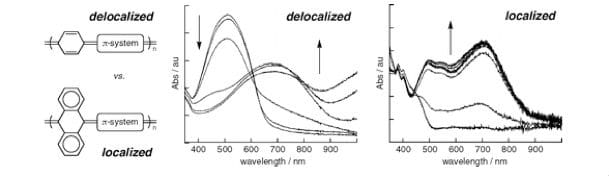
We are exploring stimuli-sensitive materials where defined photochemical processes lead to local alteration of conjugated polymer electronic or torsional properties. In this work, we have chosen materials with orthogonal degrees of aromaticity fused pendant to a polymer main chain such that transitions between aromatic and quinoidal resonance structures (as dominant contributors to observed polymer electronic properties) can lead to the selective and designed evolution of aromaticity as a function of a photocyclization event. As an initial step in this research, we studied several conjugated polymers and molecular models with varying degrees of orthogonal ring fusion spanning from phenyl linkages (no ring fusions) to 9,10-anthracenyl (two pendant ring fusions). These foundational studies currently inform our polymer designs for photoswitch elements that might similarly provoke observable differences in polymer electronic properties in a feasible manner.
Project Publications
A. M. Fraind, G. Sini, C. M. Risko, L. R. Ryzhkov, J.-L. Brédas and J. D. Tovar, “Charge Delocalization Through Benzene, Naphthalene, and Anthracene Bridges in pi-Conjugated Oligomers: An Experimental and Quantum Chemical Study,” J. Phys. Chem. B, 2013, 117, 6304-6317. DOI: 10.1021/jp401448a
Fraind, A. M.; Tovar, J. D. “A comparative survey of conducting polymers containing benzene, naphthalene and anthracene cores: interplay of localized aromaticity and polymer electronic structures,” J. Phys. Chem. B, 2010, 114, 3104-3116. DOI: 10.01021/jp9101459