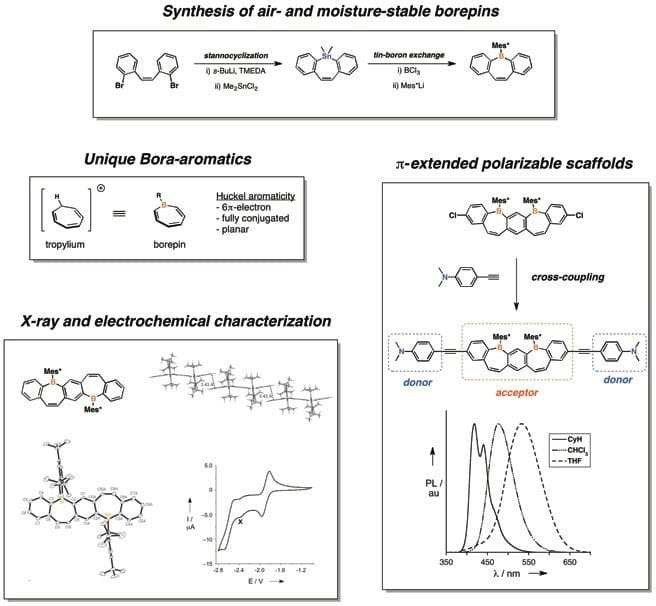
Embedding heteroatoms within the π-conjugated skeletons of organic small molecules and polymers has emerged as a powerful strategy for fine-tuning of molecular properties. The use of tricoordinate boron as the heteroatom “dopant” is of interest due to the presence of an empty p-orbital, which offers unique characteristics based on electron-deficiency. The resulting systems have exhibited promise as functional materials for a wide range of applications including selective Lewis-base sensing, non-linear optics, and n-channel charge transport.
The main challenge in realizing new organoboron materials is the stabilization of the air- and moisture-sensitive boron center. While a variety of methods have emerged to address this (including steric protection and structural constraint), we have chosen to focus on the lesser-explored concept of non-benzenoid aromaticity, such as that found in the 7-membered, boron-containing borepin ring. Our group has prepared a variety of polycyclic aromatic compounds containing fused borepin rings, all of which are air- and moisture-stable. In addition to obtaining full photophysical, electrochemical, and structural characterization of these new systems, we have developed synthetic methods to incorporate them into more complex, extended π-conjugated arrays. This has allowed fine-tuning of molecular properties through the choice of substituent and the regiochemical site of attachment. Current work in the group is focused on expanding the library of accessible borepin-based molecules to include structures which incorporate additional fused heteroaromatic rings and realizing functional devices based upon these unique aromatic motifs.
Project Publications
R. E. Messersmith, S. Yadav, M. A. Siegler, H. Ottosson and J. D. Tovar, “Benzo[b]thiophene fusion enhances local borepin aromaticity in polycyclic heteroaromatic compounds,” in the Journal of Organic Chemistry, 2017 (82) 13440-13448. DOI: 10.1021/acs.joc.7b02512
D. R. Levine, R. E. Messersmith, M. A. Siegler and J. D. Tovar, “Ring fusion isomers of dithienoborepins: perturbations of electronic structure, aromaticity and reactivity in boron-containing polycyclic heteroaromatics,” invited by the Canadian Journal of Chemistry as part of a special issue in honor of Professor Reginald Mitchell, 2017 (95) 381-389. DOI: 10.1139/cjc-2016-0493
R. E. Messersmith, M. A. Siegler and J. D. Tovar, “Aromaticity competition in differentially-fused borepin containing polycyclic aromatics,” in the Journal of Organic Chemistry, 2016 (81), 5595-5605. DOI: 10.1021/acs.joc.6b00927
R. E. Messersmith and J. D. Tovar, “Assessment of the aromaticity of borepin rings by spectroscopic, crystallographic, and computational methods: a historical overview,” J. Phys. Org. Chem., 2015, 28, 378-387 (cover article). DOI: 10.1002/poc.3422
Levine, D. R.; Siegler, M. A.; Tovar, J. D. “Thiophene-Fused Borepins as Directly Functionalizable Boron-Containing π-Electron Systems,” J. Am. Chem. Soc., 2014, 136, 7132-7139. DOI: 10.1021/ja502644e
Levine, D. R.; Caruso Jr., A.; Siegler, M. A.; Tovar, J. D. “Meta-B-entacenes: New polycyclic aromatics incorporating two fused borepin rings.” Chem. Commun., 2012, 48, 6256-6258. DOI: 10.1039/C2CC32500D
Caruso Jr., A.; Tovar, J. D. “Conjugated ‘B-entacenes’: polycylic aromatics containing two borepin rings.” Org. Lett., 2011, 13, 3106-3109. DOI: 10.1021/ol2010159
Caruso Jr., A.; Tovar, J. D. “Functionalized Dibenzoborepins as Components of Small Molecule and Polymeric Pi-conjugated Electronic Materials.” J. Org. Chem., 2011, 76, 2227-2239. DOI: 10.1021/jo2001726
Caruso Jr., A.; Siegler, M. A.; Tovar, J. D. “Synthesis of functionalzable boron-containing pi-electron materials that incorporate formally aromatic fused borepin rings. “ Angew. Chem. Int. Ed., 2010, 49, 4213-4217. DOI: 10.1002/anie.201000411