Unusual Hückel aromatics have garnered interest in the field of material science due to their interesting structural and electronic characteristics. 1,6-methano[10]annulene (M10A) is one such unusual non-benzenoid aromatic and has been studied extensively in our lab. The bridged and non-planar structure of M10A results in amorphous semiconducting material when incorporated into donor-acceptor polymers. These materials have been used to prepare organic field-effect transistors, organic photovoltaics, and thermoelectric devices. Previous work with M10A-based polymers investigated the ability of the non-planar annulene to influence torsional strain along the polymer backbone arising from alkyl chain-aromatic ring interactions. Other studies on the material tested the utility of M10A-based amorphous conjugated polymers in all-organic and hybrid organic-inorganic thermoelectric devices with a variety of dopants and nano-structures to optimize thermopower and conductivity.
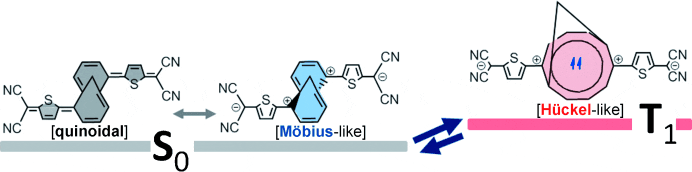
More recent research has focused on tuning the spin properties of the compound, through the synthesis of quinoidal derivatives of M10A molecules with extended conjugation. This quinoidal compound has a tendency to restore aromaticity by forming diradical species. The spin states of this diradical could be influenced by the nature of the Hückel-like or Möbius-like aromaticity within the annulene subunit.
Project Publications
B. C. Streifel, J. L. Zafra, G. L. Espejo, C. J. Gómez-García, J. Casado and J. D. Tovar, “An unusually small singlet-triplet gap in a quinoidal 1,6-methano[10]annulene due to Baird’s 4n pi-electron triplet stabilization,” Angew. Chem. Int. Ed., 2015, 54, 5888-5893 (inside cover article). DOI: 10.1002/anie.201500879
B. C. Streifel, J. F. Martinez-Hardigree, H. E. Katz, and J. D. Tovar, “Heteroaromatic variation in amorphous 1,6-methano[10]annulene-based charge-transporting organic semiconductors,” J. Mater. Chem. C., 2014, 2, 7851-7858. DOI: 10.1039/C4TC01326C
B. C. Streifel, P. A. Peart, J. F. Martinez-Hardigree, H. E. Katz and J. D. Tovar, “Torsional influences within disordered organic electronic materials based upon non-benzenoid 1,6- methano[10]annulene rings,” Macromolecules, 2012, 45, 7339-7349. DOI: 10.1021/ma301408w
G. A. Elbaz, L. M. Repka and J. D. Tovar, “Influence of annulene ratio on the electrochemical and spectroscopic properties of methano[10]annulene-thiophene random copolymers,” in ACS Applied Materials & Interfaces, 2011 (3) 2551-2556. DOI: 10.1021/am200409b
Peart, P. A.; Elbaz, G.; Tovar, J. D. “Optical and electrical properties of pi-conjugated polymers built with the 10 pi-electron methano[10]annulene ring system,” Pure Appl. Chem., 2010, 82 (4), 1045-1053. DOI: 10.1351/PAC-CON-09-10-05
Peart, P. A.; Tovar, J. D. “Expanding the realm of furan-based conducting polymers through conjugation with 1,6-methano[10]annulene,” Macromolecules, 2009, 42 (13), 4449-4455. DOI: 10.1021/ma9006494
Peart, P. A.; Repka, L. M.; Tovar, J. D. “Emerging Prospects for Unusual Aromaticity in Organic Electronic Materials: The Case for Methano[10]annulene.” Eur. J. Org. Chem. 2008, 2193–2206. DOI: 10.1002/ejoc.200800533
Peart, P. A.; Tovar, J. D. “Methano[10]annulene revisited: extended delocalization through conjugated polymers bearing larger Hückel aromatics.” Org. Lett. 2007, 9 (16), 3041-4. DOI: 10.1021/ol071062y