Release and transformation of additives from polymers in aqueous media
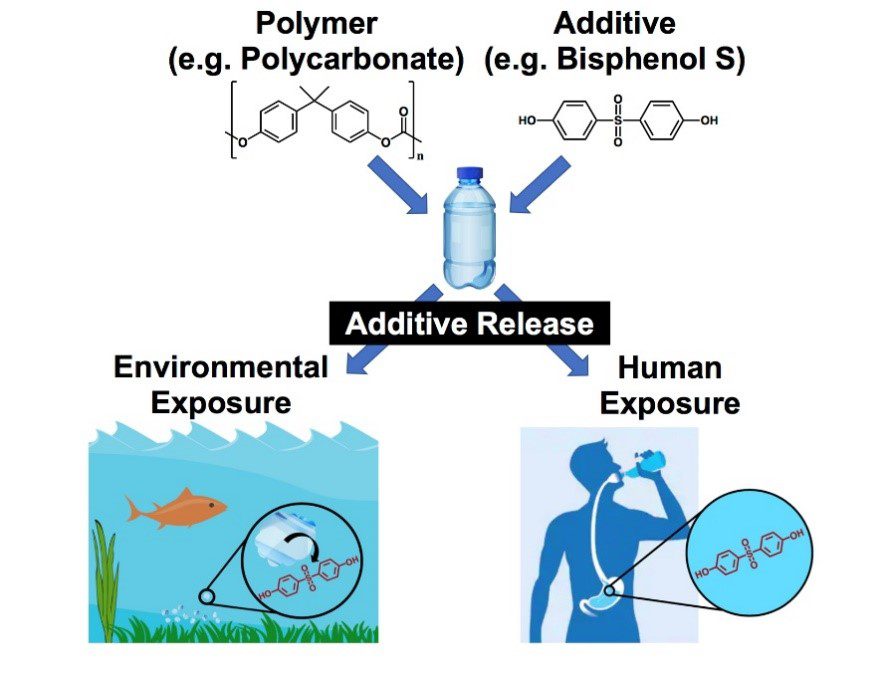
Chemical additives, such as benzophenones and bisphenols, are blended into polymers to create plastics with enhanced functionality and durability. When a plastic reaches the end of its consumer lifetime and is discarded, physical (abrasion) and chemical (weathering) degradation processes result in the leaching of additives to the surrounding environment. Despite an ever-growing presence in plastic products, a detailed understanding of the factors controlling the rate of additive release and potential toxic transformations in aqueous environments remains undetermined. To address this challenge, we are investigating the release and transformations of polymer additives in a variety of environmental degradation scenarios. Our approach is to fabricate additive-modified polymer composites and expose them to accelerated weathering conditions, such as high intensity UV irradiation, aggressive oxidation, and thermal cycling. We utilize a combination of analytical techniques, including LC-HRMS, TOF-SIMS, and GC-MS, to identify the fundamental release kinetics and reaction mechanisms of additives. This work is being conducted in collaboration with Professor Carsten Prasse at the Johns Hopkins Bloomberg School of Public Health and with Professor Jim Ranville at the Colorado School of Mines.
Impact of chemical modification on the dispersion and biodegradability of nanocellulose

Nanocellulose is a naturally produced biopolymer which possesses the desirable properties of natural abundance, biocompatibility, biodegradability, and mechanical strength. As such, nanocellulose has a range of applications in the material, biomedical, and food industries which leverage these properties in nano-enabled products. For use in these fields, nanocellulose often needs to be modified via hydrophobic coating or chemical functionalization of hydroxyl groups on its surface to reduce aggregation and improve the transfer of its properties to the product. The effect that these surface modifications have on the biodegradation of nanocellulose is of particular importance to identify, since the low environmental impact of nanocellulose is one of its most attractive properties. The Fairbrother group is currently seeking to identify the impact that different hydrophobic ether and ester groups have on the anaerobic and aerobic biodegradability of nanocellulose. This research utilizes a range of techniques including organic synthesis, material characterization (ATR-FTIR, XPS, SEM, EDS), and microbial degradation to holistically assess the environmental transformations of functionalized nanocellulose. This project is being carried out as part of our work with the NSF funded Center for Sustainable Nanotechnology (CSN).
Sustainable plant nutrient delivery through biodegradable polymer nanocomposites
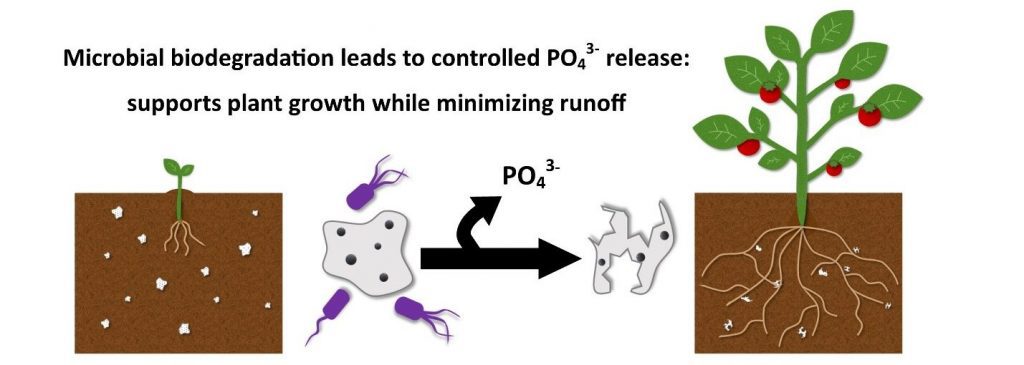
Phosphorus is an important but often limiting nutrient required for plant growth and traditional fertilizers are often heavily applied to agricultural soils to maintain healthy crops. However, much of the phosphorus in traditional fertilizers is lost as runoff, which migrates to surface waters and causes eutrophication and degradation of aquatic environments. Polymer nanocomposites made from biodegradable polymers and phosphate-containing nanoparticles can serve as an environmentally benign alternative to traditional fertilizers. As naturally occurring soil microbes biodegrade the polymer, nanoparticles will be released from the polymer matrix, providing nutrients in a targeted and controlled fashion to plants over time. We are also exploring how different combinations of polymers and nanoparticles can provide temporal control and tunability for use in various crops. This project is being carried out as part of our work with the NSF funded Center for Sustainable Nanotechnology (CSN) in collaboration with the Connecticut Agricultural Experiment Station.
Photochemical transformations of Carbon Dots
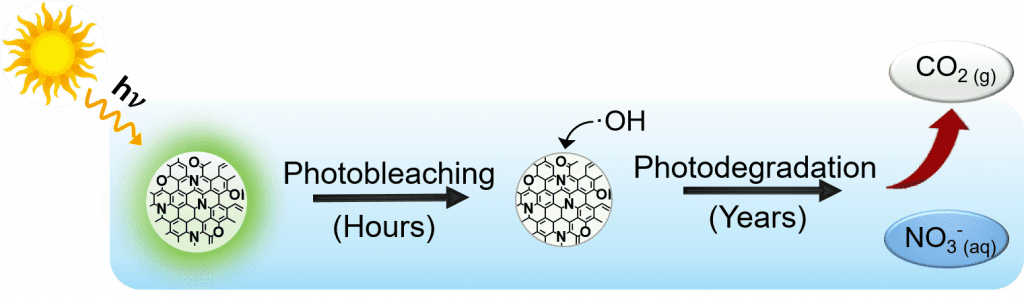
Carbon dots (CDs) are fluorescent carbon nanomaterials with optical properties similar to semiconductor quantum dots. However, CDs do not contain toxic heavy metals and can be made from a variety of renewable materials like agricultural waste or discarded plastic bags. Increased use of CDs will lead to their increased presence in the natural environment, which necessitates a better understanding of the chemical transformations that CDs undergo in response to exposure to light. We have found that CDs synthesized by a bottom up approach are rapidly photobleached by the effects of direct sunlight over the course of several hours, but are subsequently photodegraded by the hydroxyl radicals formed by indirect photolysis over the course of many years. By studying the CD photobleaching process in more detail we also hope to provide valuable new insights into those structural elements associated with the CD fluorophores. Currently, the exact mechanism of CD photoluminescence is unclear and understanding the underlying chemical transformations that occur to CDs as they lose their fluorescence could inform the design of next generations CDs, with improved optical properties more able to replace semiconductor quantum dots in applications such as sensing, bioimaging, and renewable energy. This project is being carried out as part of our work with the NSF funded Center for Sustainable Nanotechnology (CSN).
Modification of Polysaccharides for Nutrient Delivery in Sustainable Agriculture
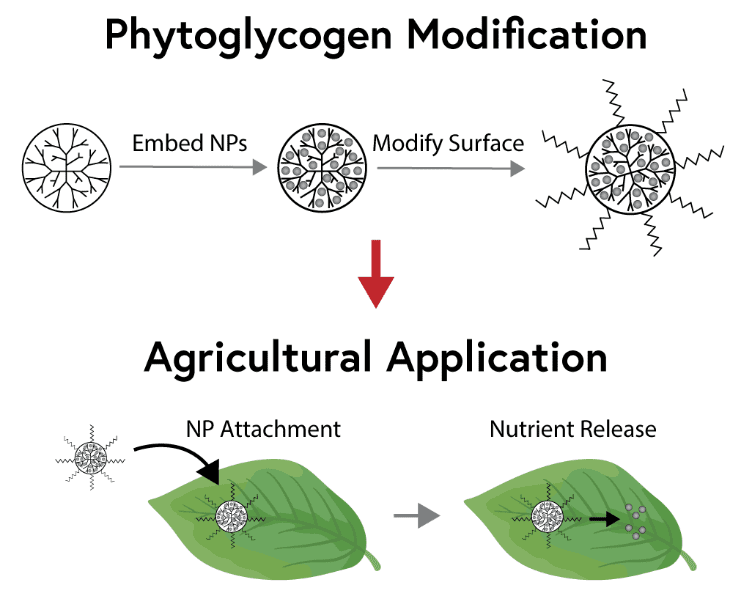
Conventional fertilizers used in agriculture can be lost to soil complexation, volatilization, or runoff into groundwater, resulting in negative economic and environmental impacts. Alternatively, next-generation targeted delivery systems can offer increased agricultural yields while limiting waste and pollution. Phytoglycogen nanoparticles (PhG NPs) are renewable polysaccharides naturally produced in corn that offer great potential as a nutrient delivery platform. The dendritic structure of PhG NPs facilitates embedding of nutrient materials within the branches, while the surface chemistry can be modified to optimize PhG NP binding to plant surfaces (e.g. hydrophobic leaves or roots). In this work, we are embedding zinc phosphate and zinc oxide nutrients within the PhG NPs and functionalizing PhG surfaces to increase targeted adhesion to plant leaves. This work is being conducted in collaboration with Mirexus Biotechnologies as part of the Center for Sustainable Nanotechnology.
Impact of Surface Oxidation of Black Carbon in the Atmosphere
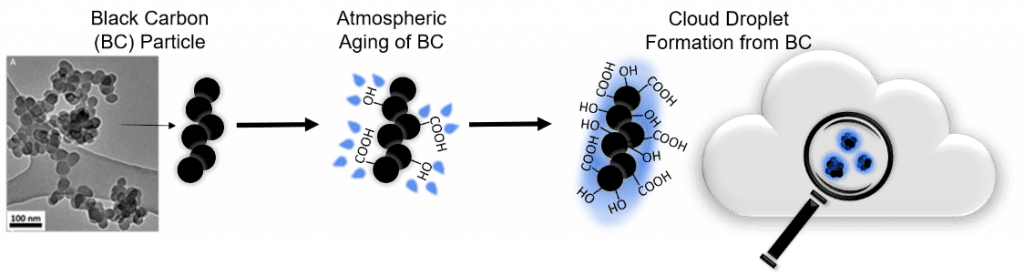
This project aims to develop a molecular level understanding of how the chemical composition of the surface of black carbon (BC) particles, released into the atmosphere by combustion processes (e.g. burning wood), influences its ability to form rain droplets. These particles are of concern due to their small size and heterogeneity caused by emissions from various sources and ignition processes, which complicates the study of their environmental impact. The surface chemistry of black carbon particles is complex and varies considerably due to differences in the carbon source, combustion conditions and subsequent oxidation (aging) in air. Although BC particles are modeled as hydrophobic and insoluble, the effect of hydrophilic modification by adding functional groups (e.g. -COOH) on the surface has not been widely studied. This project seeks a better understanding of how surface reactivity influences black carbon’s ability to form droplets. We utilize oxidation methods to alter the surface chemistry of the BC particles and numerous surface characterization and analytical techniques to analyze the particles. This project is in collaboration with Professor Akua Asa-Awuku at the University of Maryland.
