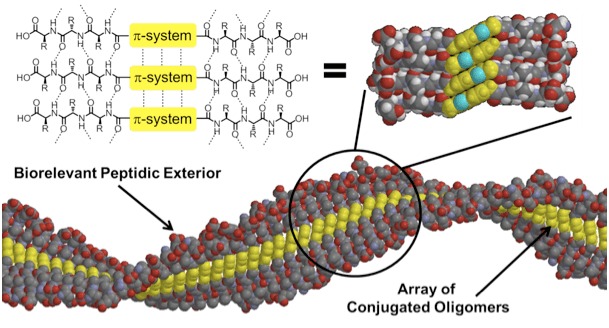
Utilizing intermolecular, non-covalent interactions to prepare supramolecular semiconducting materials allows for the formation of systems in the micron-size regime, which are difficult to achieve with other techniques. We have sought to exploit the hydrogen-bond mediated self-assembly of peptides to create these types of materials by embedding π-conjugated subunits directly in the peptide backbone. In addition to facilitating supramolecular organization, the peptidic scaffolds also render the materials aqueous processable and give them the potential to be used in biologically relevant applications.
Synthetic Methods
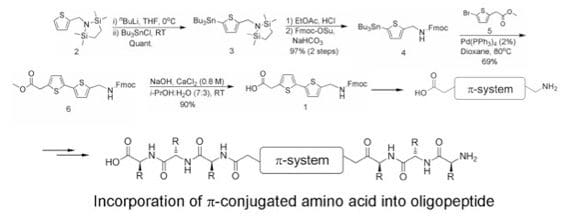
Our group has developed three robust methods to functionalize the interior of the peptide backbone with semiconductive π-conjugated subunits. The first involves the synthesis of π-conjugated “amino acids” which can be incorporated into the peptide during solid-phase peptide synthesis.
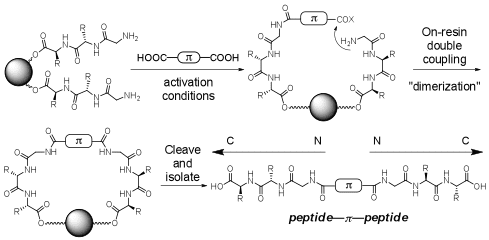
The second method involves a solid-phase double amidation “dimerization” utilizing a dicarboxylic acid π-conjugated analogue. This method allows for the rapid synthesis of “symmetrical” peptides.
The most recent technique involves the use of palladium catalyzed cross-coupling on the solid-phase. This method allows for the inclusion of more complex π-conjugated oligomers, without requiring lengthy solution-phase syntheses of the insoluble subunits.
Characterization
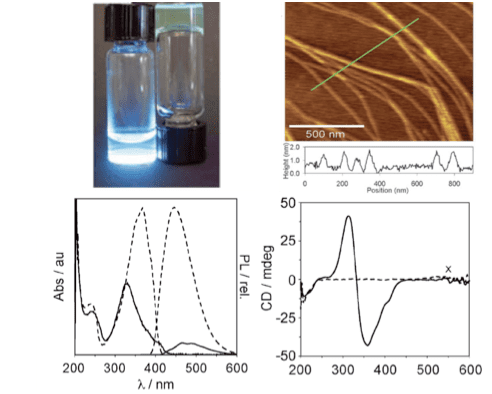
Spectroscopic techniques such as UV-vis, photoluminescence, and circular dichroism are utilized to study the assembly of the π-conjugated peptides. Furthermore, microscopy (TEM, SEM), allow us to visualize the assembled 1-D nanostructures, which typically have lengths in the micron-size regime.
Continued work on bioelectronic self-assembling peptides includes the investigation into structure-function relationships between chromophore and peptide sequence, utilizing various spectroscopy and microscopy techniques. These studies will enable us to elucidate how chromophore orientation can be used to govern the electronic properties of the supramolecular peptide aggregates.
Biomaterial Applications
The amino acids that insulate our π-conjugated systems serve more function than just directing the self-assembly of nanofibers; they also provide a bioactive interface for interaction with biological systems. Upon assembly, the π-chromophore is internalized and only the natural amino acids are exposed on the exterior of the nanofiber. By utilizing primary amino acid sequences capable of cell adhesion, we have the potential to control and/or monitor cellular events like attachment, growth, migration, or differentiation. In vitro experiments are underway to investigate the biocompatibility of and cellular interaction with these soft nanobiomaterials.
Project Publications
S. S. Panda, H. E. Katz and J. D. Tovar, “Solid-state electrical applications of protein and peptide-based nanomaterials,” invited by Chemical Society Reviews (part of a special issue on “Peptide and Protein Nanotechnology”), submitted.
J. D. Tovar, “Photon management in supramolecular peptide nanomaterials,” invited by Bioinspiration and Biomimetics (part of a special issue on “Biophotonics and biologically inspired photonics”), 2018 (13) 015004. DOI: 10.1088/1748-3190/aa9685
Y. Zhou, B. Li, S. Li, H. A. M. Ardoña, W. L. Wilson, J. D. Tovar and C. M. Schroeder, “Concentration-driven assembly and sol-gel transition of pi-conjugated oligopeptides,” in ACS Central Science, 2017 (3) 986-994. DOI: 10.1021/acscentsci.7b00260
T. S. Kale, J. E. Marine and J. D. Tovar, “Self-assembly and associated photophysics of dendron-appended peptide-pi-peptide triblock macromolecules,” in Macromolecules, 2017 (50) 5315-5322. DOI: 10.1021/acs.macromol.7b00821
H. A. M. Ardoña, E. R. Draper, F. Citossi, M. Wallace, L. Serpell, D. J. Adams, J. D. Tovar, “Kinetically controlled coassembly of multichromophoric peptide hydrogelators and the impacts on energy transport,” in the Journal of the American Chemical Society, 2017 (139) 8685-8692. DOI: 10.1021/jacs.7b04006
H. A. M. Ardoña, T. S. Kale, A. Ertel and J. D. Tovar, “Non-resonant and local field effects on the photophysics of oligo(phenylenevinylene) segments within peptidic nanostructures,” in Langmuir, 2017 (33) 7435-7445. DOI: 10.1021/acs.langmuir.7b01023
A. M. Sanders, T. S. Kale, H. E. Katz and J. D. Tovar, “Solid-phase synthesis of self-assembling multivalent pi-conjugated peptides,” in ACS Omega, 2017 (2) 409-419. DOI: 10.1021/acsomega.6b00414
B. Li, S. Li, Y. Zhou, H. A. M. Ardoña, L. R. Valverde, W. L. Wilson, J. D. Tovar and C. M. Schroeder, “Non-equilibrium self-assembly of pi-conjugated oligopeptides in solution,” in ACS Applied Materials & Interfaces, 2017 (9) 3977-3984. DOI: 10.1021/acsami.6b15068
T. S. Kale and J. D. Tovar, “Synthesis and Evaluation of Self—Assembled Nanostructures of Peptide-pi chromophore Conjugates,” invited by Methods in Molecular Biology, in press.
W. Liyanage, H. A. M. Ardoña, H.-Q. Mao and J. D. Tovar, “Cross-linking approaches to tune the mechanical properties of peptide-pi-electron based hydrogels,” invited by Bioconjugate Chemistry, 2017 (28) 751-759. DOI: 10.1021/acs.bioconjchem.6b00593
T. S. Kale and J. D. Tovar, “Regulation of Peptide-π-Peptide Nanostructure Bundling: The Impact of “Cruciform” π-Electron Segments,” invited as part of a Symposium-in-Print in honor of the retirement of Professor Gary H. Posner, Tetrahedron, 2016 (72), 6084-6090. DOI: 10.1016/j.tet.2016.07.064
A. M. Sanders, T. J. Magnanelli, A. E. Bragg and J. D. Tovar, “Photoinduced electron transfer within supramolecular donor-acceptor peptide nanostructures under aqueous conditions,” J. Am. Chem. Soc. 2016, 138, 3362-3370. DOI: 10.1021/jacs.5b12001
H. A. M. Ardoña and J. D. Tovar, “Peptide pi-electron conjugates: organic electronics for biology?” Invited cover article by Bioconjugate Chemistry, 2015, 26, 2290-2302. DOI:10.1021/acs.bioconjchem.5b00497
K. Besar, H. A. M. Ardoña, J. D. Tovar and H. E. Katz, “Demonstration of hole transport and voltage equilibration in self-assembled pi-conjugated peptide nanostructures using field-effect transistor architectures,” ACS Nano, 2015, 9, 12401-12409. DOI: 10.1021/acsnano.5b05752
H. A. M. Ardoña, K. Besar, M. Togninalli, H. E. Katz and J. D. Tovar, “Sequence-dependent mechanical, photophysical and electrical transport properties of pi-conjugated peptide hydrogelators,” J. Mater. Chem. C (part of a special themed issue on bioelectronics), 2015, 3, 6505-6514. DOI: 10.1039/C5TC00100E
H. A. M. Ardoña and J. D. Tovar, “Energy transfer within responsive pi-conjugated peptide-based coassembled nanostructures in aqueous environments,” Chem. Sci., 2015, 6, 1474-1484. DOI: 10.1039/C4SC03122A
B. D. Wall, Y. Zhou, S. Mei, H. A. M. Ardoña, A. L. Ferguson and J. D. Tovar, “Variation of formal hydrogen bonding networks within electronically delocalized pi-conjugated oligopeptide nanostructures,” Langmuir, 2014, 11375-11385. DOI: 10.1021/la501999g
B. D. Wall, A. E. Zacca, A. M. Sanders, W. L. Wilson, A. L. Ferguson and J. D. Tovar, “Supramolecular polymorphism: Tunable electronic interactions within pi-conjugated peptide nanostructures dictated by primary amino acid sequence,” Langmuir, 2014, 30, 5946-5956. DOI: 10.1021/la500222y
A. M. Sanders and J. D. Tovar, “Solid-phase Pd-catalyzed cross-coupling methods for the construction of π-conjugated peptide nanomaterials,” Supramol. Chem., 2014, 26, 259-266. DOI:10.1080/10610278.2013.852675
J. D. Tovar, “Supramolecular construction of optoelectronic biomaterials,” Acc. Chem. Res., 2013, 46, 1527-1537. DOI: 10.1021/ar3002969
A. M. Sanders, T. J. Dawidcyzk, H. E. Katz and J. D. Tovar, “Peptide-based supramolecular semiconductor nanomaterials via Pd-catalyzed solid-phase ‘dimerizations’,” ACS Macro Lett., 2012, 1, 1326-1329. DOI:10.1021/mz3004665
S. R. Diegelmann, N. Hartman, N. Markovic and J. D. Tovar, “Synthesis and alignment of discrete polydiacetylene-peptide nanostructures,” J. Am. Chem. Soc., 2012, 134, 2028-2031. DOI: 10.1021/ja211539j
B. D. Wall, S. R. Diegelmann, S. Zhang, T. J. Dawidczyk, W. L. Wilson, H. E. Katz, H.-Q. Mao and J. D. Tovar, “Aligned macroscopic domains of optoelectronic nanostructures prepared via the shear flow assembly of peptide hydrogels,” cover article in Advanced Materials, 2011 (23) 5009-5014. DOI: 10.1002/adma.201102963
Vadehra, G. S.; Wall, B. D.; Diegelmann, S. R.; Tovar, J. D. “On resin dimerization incorporates a diverse array of pi-conjugated functionality within aqueous self-assembling peptide backbone.” Chem. Commun., 2010, 46, 3947-3949. DOI: 10.1039/c0cc00301h
Diegelmann, S. R.; Gorham, J. M.; Tovar J. D. “One-dimensional optoelectronic nanostructures derived from the aqueous self-assembly of π-conjugated oligopeptides.” J. Am. Chem. Soc. 2008, 130 (42), 13840-1. DOI: 10.1021/ja805491d