Electron Stimulated Surface Reactions of Organometallic Precursors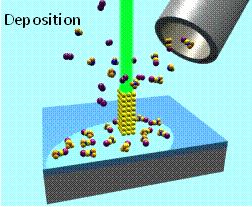
Electron beam induced deposition (EBID) of metal nanostructures from organometallic precursors.–courtesy of Ivo Utke (EMPA, Switzerland)
Electron beam induced deposition (EBID) uses the electron stimulated decomposition of volatile organometallics under low vacuum conditions to create nanostructures in a minimally-invasive, next-generation, maskless lithographic process. As a tool for nanofabrication, EBID offers an attractive and unique combination of capabilities including high spatial resolution and the flexibility to deposit free-standing three-dimensional structures on non-planar surfaces (Figure right). Despite the inherent advantages of EBID the deposits often contain
Electron beam induced deposition (EBID) uses the electron stimulated decomposition of volatile organometallics under low vacuum conditions to create nanostructures in a minimally-invasive, next-generation, maskless lithographic process. As a tool for nanofabrication, EBID offers an attractive and unique combination of capabilities including high spatial resolution and the flexibility to deposit free-standing three-dimensional structures on non-planar surfaces (Figure right). Despite the inherent advantages of EBID the deposits often contain unacceptably high level of organic contamination. These impurities negatively impact the properties (e.g. resistivity) and function (e.g. catalytic activity) of EBID nanostructures. Most of the contamination emanates from the electron stimulated decomposition of the ligand architecture that surrounds the central metal atom in the organometallic precursor. In the past several years we have used an array of surface analytical techniques (X-ray Photoelectron Spectroscopy, Mass Spectrometry, and Infrared Spectroscopy) to interrogate the sequence of electron stimulated bond breaking processes that occur within nanometer scale thick films of EBID precursors (e.g. MeCpPtMe3, Pt(PF3)4 and W(CO)6) under UHV conditions. Results from these studies have revealed that there a well-defined sequence of electron stimulated reactions which govern the decomposition of surface bound organometallic precursors which depend on the ligand architecture. This work has involved collaborations with Kees Hagen at TU Delft. Recently we have started to work with Professor Lisa McElwee-White at the University of Florida to use the “rules” of electron stimulated reactions we have uncovered in an effect to design organometallic precursors specifically for EBID.
Properties of Size Selected Clusters on Surface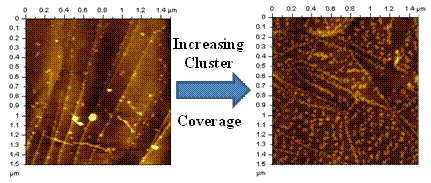
Influence of cluster coverage on the two-dimensional structure of size-selected molybdenum oxide clusters (resolved as lighter features) deposited on a HOPG substrate.
Small, gas-phase clusters often exhibit novel electronic, magnetic, and catalytic properties compared to bulk materials having the same compositions. Such properties are strongly dependent on cluster size, even to the point where the addition or removal of a single atom can vastly alter a given property. Description: Picture1.emf Influence of cluster coverage on the two-dimensional structure of size-selected molybdenum oxide clusters (resolved as lighter features) deposited on a HOPG substrate. To harness their finite-size properties for potential applications in the macroscopic world, however, most clusters will either have to reside on surfaces or be incorporated into materials. For this reason, it is important to understand how size selected clusters behave on the surface of solid substrates. In these experiments, gas phase clusters are first generated in the gas phase using a magnetron sputtering source before being size selected by passing them through a magnetic sector. The size selected clusters are subsequently soft landed onto a HOPG surface and imaged by either scanning tunneling or atomic force microscopy (STM or AFM). The AFM images (right) shows how the two-dimensional structure of size-selected molybdenum oxide clusters deposited on HOPG vary as a function of cluster coverage. At comparatively low coverages the clusters preferentially nucleate at the HOPG step edge before forming larger aggregates on the HOPG terraces at higher cluster coverages. Future studies include plans to study the catalytic properties of size-selected metal oxide clusters. These studies are conducted under the supervision of Professor Kit Bowen in the Chemistry Department at Johns Hopkins University.
Etching of Carbonaceous Contaminant Films by Atomic Hydrogen

Extreme Ultraviolet (EUV) lithographic tool for patterning photoresists
Extreme ultraviolet lithography (EUVL) is an enabling technology that the microelectronics industry intends to introduce for high volume production of leading- edge semiconductor integrated circuits beginning in the 2014 time frame. In EUVL 13.5nm light is reflected at near-normal incidence by multilayer mirrors that consist of precisely engineered Mo/Si multilayer coatings before patterning the photoresist, as shown in the Figure (right). Presently, the deposition of carbonaceous layers on the mirror surfaces is one of the key problems that must be overcome to attain commercial viability of EUVL. These layers, formed by the radiation-induced activation of adsorbed organic molecules, diminish the reflectivity of the multilayer coatings on the EUV mirrors, reducing the throughput of the EUV lithography tools. In addition, carbonaceous layers also cause distortion of the EUV radiation wavefronts, compromising the ability to print uniform features. In collaboration with Tom Lucatorto , Shannon Hill and Nadir Faradzhev at the National Institute of Standards and Technology (NIST) in Gaithersburg, MD we are exploring the potential to use atomic hydrogen (AH) to remove carbonaceous layers from EUV mirrors without compromising the atomically smooth mirror surface. Our research includes studying the effects that chlorine, phosphorous and sulfur atoms have on the etching of carbonaceous layers by AH as well as the potential for electron beam induced deposition (EBID) to create contaminant films that mimic the properties of those created on EUV mirrors.
DR. HOWARD FAIRBROTHER ALSO MAINTAINS JHU SURFACE ANALYSIS LAB
