Molecular photoswitches undergo large structural changes following activation with light that alter their electronic and optical properties. Photoswitches have garnered significant attention in recent years, primarily due to their tantalizing potential for use as optical data storage, molecular electronics, biological imaging and molecular machines. However, there are many challenges associated with the design and implementation of such photoswitches since it is necessary that they are both robust and fatigue-resistant whilst also exhibiting desirable spectral and photophysical properties, such as visible light absorption and high conversion efficiencies. An understanding of the underlying photophysical mechanisms is therefore extremely valuable when it comes to the design and manipulation of useful photoswitchable materials. In the Bragg lab, we utilize an array of time-resolved spectroscopic methods (from steady-state to ultrafast) coupled with computational studies in order to understand how molecular structure affects the accessibility, efficiency and the rate of excited-state relaxation mechanisms present in photo-activated switches.
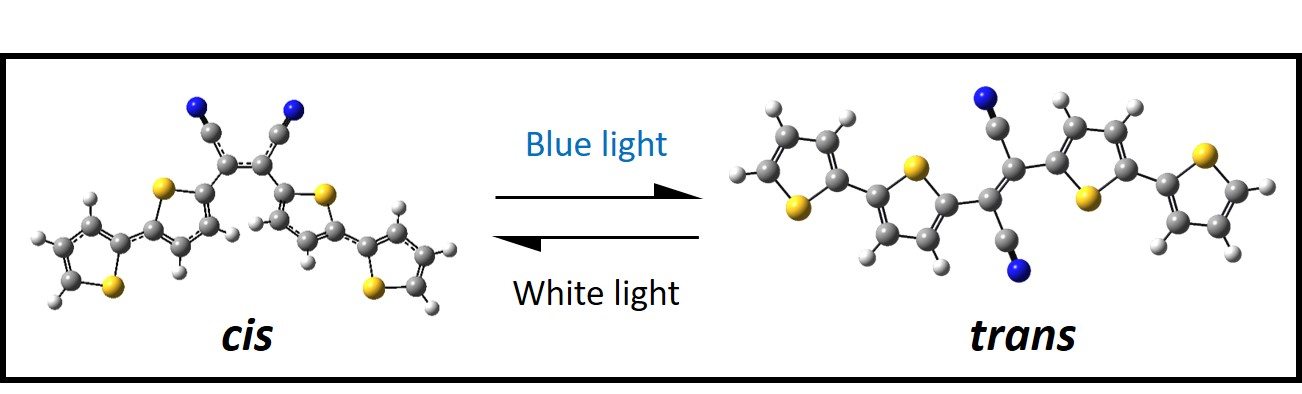
Recent efforts have been devoted to exploring the isomerization and excited state dynamics of the diarylethene photoswitch, bis(dithienyl)-1,2-dicyanoethene, 4TCE. Contrary to most diarylethene systems, 4TCE’s photoswitching mechanism operates exclusively via E/Z isomerization as opposed to the conventional 6π photocyclization that is more commonly observed. Additionally, the isomerization mechanism available to each isomer takes place on excited states of differing spin multiplicity. Currently we are investigating the excited state relaxation mechanisms exhibited specifically by the cis- isomer as a function of excitation energy to better understand the deactivation pathways available to cis-4TCE and to better understand why this photoswitch is seemingly so resilient to the more prototypical photocyclization mechanism mentioned previously.
We are also collaborating with the Tovar Lab at JHU to investigate cyclization and ring-opening dynamics in a series of novel, cross-conjugated diarylethene photo-switches. With synthetic modification of either the bridging groups or the pendant moieties it is possible to drastically impact ultrafast photochemical dynamics. This ongoing project involves exploring the effect of cross-conjugation on photoswitch behavior with the end goal of identifying photoswitchable subunits that could be embedded within conjugated polymers/oligomers. Specifically, we are utilizing ultrafast transient absorption spectroscopy to identify competing relaxation pathways from excited states, and how increasing or decreasing the extent of conjugation affects the propensity for photoswitching.